Build A Tips About How To Increase Concentration Of A Solution
Concentration can be increased by allowing some of the.
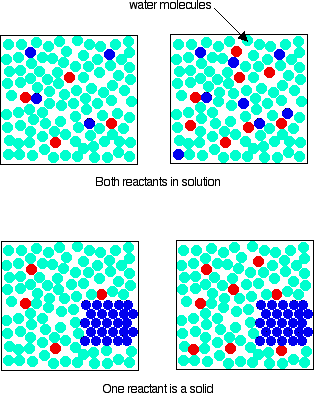
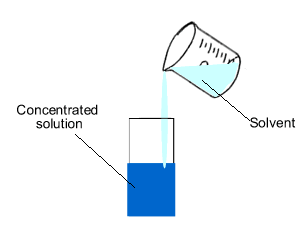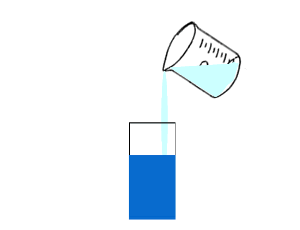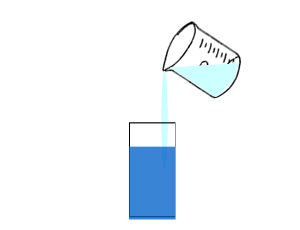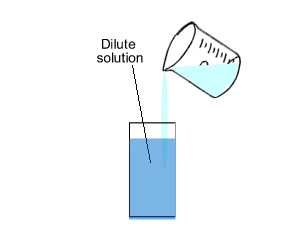
How to increase concentration of a solution. There are various methods of expressing the concentration of a solution. When a solute is added to a solvent the freezing point of the. When more solvent is added to a solution, what happens to the concentration of the solution will it increase or decrease answer:
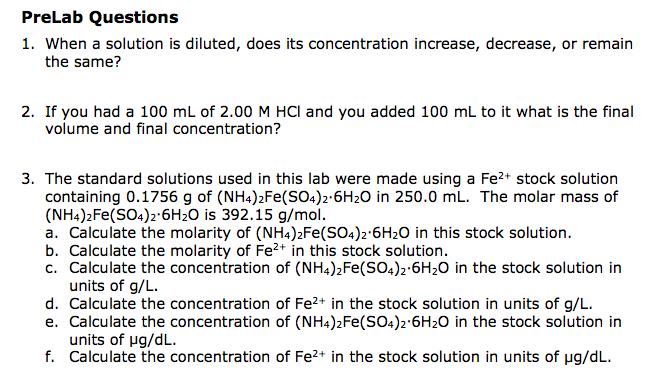
Ways to improve concentration, include brain games, meditation, music, and more. If you're finding it hard to focus and these tips don't help, consider asking a doctor. (c1) (v1) = (c2) (v2) where:
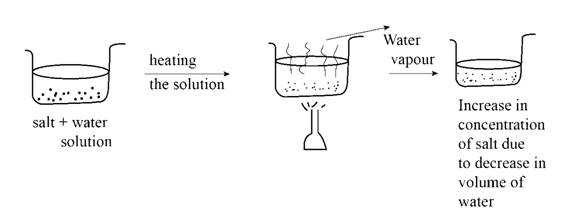
Applicable to % v/v and % w/w. The simplest way to change the concentration would be to change the amount of solute or solvent in the solution. If we want to increase the concentration, we can either (i) reduce the volume of the solution by evaporating or stripping off the solvent (and this is rather hard for aqueous solutions);
This short video challenges students to figure out how to make the color of a solution more concentrated without adding more food coloring! First, determine how many moles are present in 3 grams of kcl. Methods of expressing the concentration of solution.
If we want to increase the concentration, we can either (i) reduce the volume of the solution by evaporating or. You will usually see chemists working with the number of. Concentration is the removal of solvent, which increases the concentration of the solute in the.
C1 = the starting concentration v1 = the. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table. The equation is shown here, and in spoken terms is often referred to as c one v one equals c two v two.