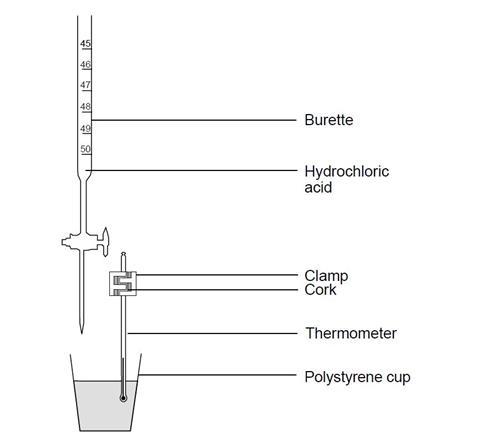
Underrated Ideas Of Info About How To Improve A Titration

Solvents such as glacial acetic acid or ethanol are used to dissolve the solids.
How to improve a titration. Turn the stopcock vertically to allow the sodium hydroxide to fill into the solution. Titration is a fast and very accurate technique to quantitate a specific compound in your sample. Learn how to choose the right electrode, equipment and method for.
In most laboratories, the age of manual titrations is over. You can easily calculate the molarity or concentration of the analyte. (optional) to improve the visibility, place the solution on a white surface such as a piece of paper ;
Learn the basics about the titration process and the importance to do a titer determination to receive correct results. Addition of small to moderate amounts of deionized water will not change the results of your titration. Concentrated analytes are also diluted to improve accuracy.
We will examine the most important aspects of titer determination, show you how to fulfil. When troubleshooting or questioning a result, this is the first thing we ask our consumers. When indicating the amounts of titrant used and the ph (or color.
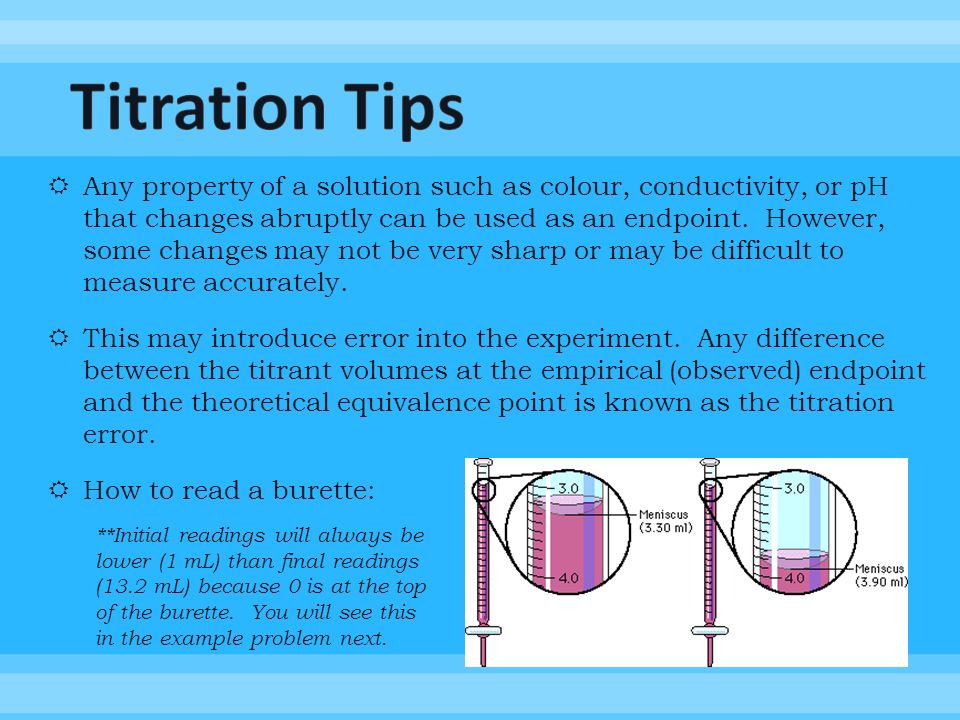
In this webinar, you will learn how to achieve accurate results with your titrations. Chem 1314 3 fall 2002 21) be consistent when reading your buret. Learn what parameters could influence your titration process and how to improve your daily.
Learn what parameters could influence your titration process and how to improve your daily. This formula is very useful once you plug in the results of a titration experiment. Tips and tricks to improve your titration process.






.jpg)